The 2025 European Society for Medical Oncology (ESMO) Annual Meeting will be held from October 17–21 in Berlin, Germany. As one of the most influential oncology conferences in Europe, it will showcase cutting-edge theories and advancements in the field of oncology worldwide.
In recent years, an increasing number of studies from China have been featured at this event, with some even selected as Late-Breaking Abstracts (LBA)-a prestigious category reserved for groundbreaking research.
For instance, XTR008, an innovative radiopharmaceutical conjugate (RDC) independently developed by Sinotau Pharmaceutical, has been selected for the LBA Proffered Paper Session, with its full Phase III clinical trial results set to be unveiled globally on October 18.
XTR008 is a ¹⁷⁷Lu-labeled somatostatin receptor (SSTR)-targeting radioligand, sharing the same target as Novartis' blockbuster drug Lutathera.
The selected study (XT-XTR008-3-01) is a Phase III trial comparing ¹⁷⁷Lu-Dotatate (XTR008) with high-dose long-acting octreotide (LAR) in patients with well-differentiated, grade 1-2 gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Interim analysis results demonstrated that XTR008 exhibits significant efficacy and safety, with key metrics such as progression-free survival (PFS) and objective response rate (ORR) significantly outperforming the high-dose LAR control group. Moreover, its performance was comparable or even superior to that observed in Lutathera's Phase III NETTER-1 trial, suggesting Best-in-Class (BIC) potential.
Based on these interim results, Sinotau Pharmaceutical submitted a New Drug Application (NDA) to China's National Medical Products Administration (NMPA) in March 2025, which was accepted in April 2025. The drug is expected to be approved and launched in 2026.
As a leading radiopharmaceutical company in China, Sinotau filed for an IPO on the Hong Kong Stock Exchange in May 2025, potentially becoming the first radiopharmaceutical firm listed under the HKEX's Chapter 18A.
Beyond XTR008, Sinotau has a robust pipeline of radiopharmaceutical candidates, including:
XTR006: An ¹⁸F-labeled tau-targeting PET diagnostic radioligand (developed in collaboration
XTR004: A self-developed PET-MPI tracer
XTR003: A PET tracer for myocardial fatty acid metabolism imaging
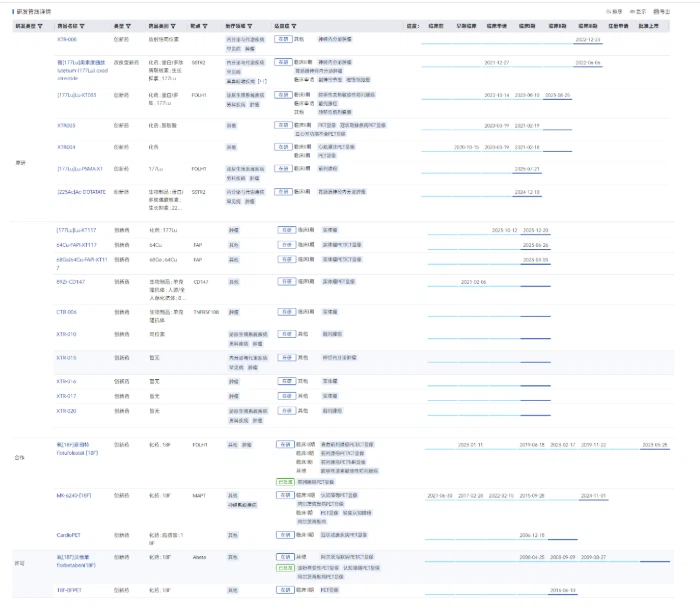
Image source: Pharma Intelligence - Global Drug Analysis System
Conclusion
The ESMO Annual Meeting is one of the most influential international conferences in oncology, and the LBA category represents the highest level of research, reserved only for breakthrough studies that could reshape clinical practice or disease understanding.
This year, 23 studies from Chinese researchers were selected as LBAs-a notable increase from just 7 last year, reflecting the growing prominence of China's innovative drugs on the global stage.
References:
1.https://mp.weixin.qq.com/s/2D6a5qdCYtb4S7LaWALXAg
2.https://mp.weixin.qq.com/s/4lXfy-3u40lKSgWKqf_Uyw